Welcome to the ECHO Connector! The Environmental influences on Child Health Outcomes (ECHO) Program is a research program in the Office of the Director at the NIH with the mission to enhance the health of children for generations to come. The ECHO Connector will keep you informed of program news and our latest research findings.
Message from Matt
A message from the ECHO Director Matthew W. Gillman, MD, SM

Happy New Year! In this first issue of the ECHO Connector for 2024, I highlight how the entire scientific community can use ECHO data to explore critical scientific questions in child health.
De-identified data from the ECHO Cohort are available to any qualified researcher through the Eunice Kennedy Shriver National Institute for Child Health and Development (NICHD) Data and Specimen Hub (DASH). Over 60,000 ECHO Cohort participants have generously shared their data on this platform, including information about demographics, early development, environmental exposures, pregnancy and birth, and public health crises, as well as child health outcomes. I hope that a large number of scientists will use these data for teaching and research. I think they will be particularly fruitful for researchers in training, who often look for meaningful data to answer their research questions in a relatively short period of time.
This month’s spotlight story highlights one example of the richness of ECHO Cohort data available in DASH—data on nutrition. We know from animal experiments and smaller studies of human populations that diet early in development can have long-lasting consequences. Now, longitudinal ECHO data on over 25,000 pregnancies and over 25,000 children, from 34 states, are ready for researchers to explore questions of diet and child health in a nationwide sample. You can learn more below about the publication that describes this nutritional resource and how researchers can request access to ECHO Cohort data in DASH.

—Matthew W. Gillman, MD, SM
Back to top
ECHO Data Available
NEW ECHO Data Now Available as Resource for Scientific Community
The goal of the ECHO Program is to understand the effects of a broad range of early environmental influences on child health and development. One key way that we do this is by providing de-identified data about ECHO Cohort participants so that the larger scientific community can discover new insights about prenatal, perinatal and pediatric health.
As of January 2024, researchers have access to even more of this ECHO data.
The second release of de-identified public-use data from the ECHO Cohort is now available through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH).
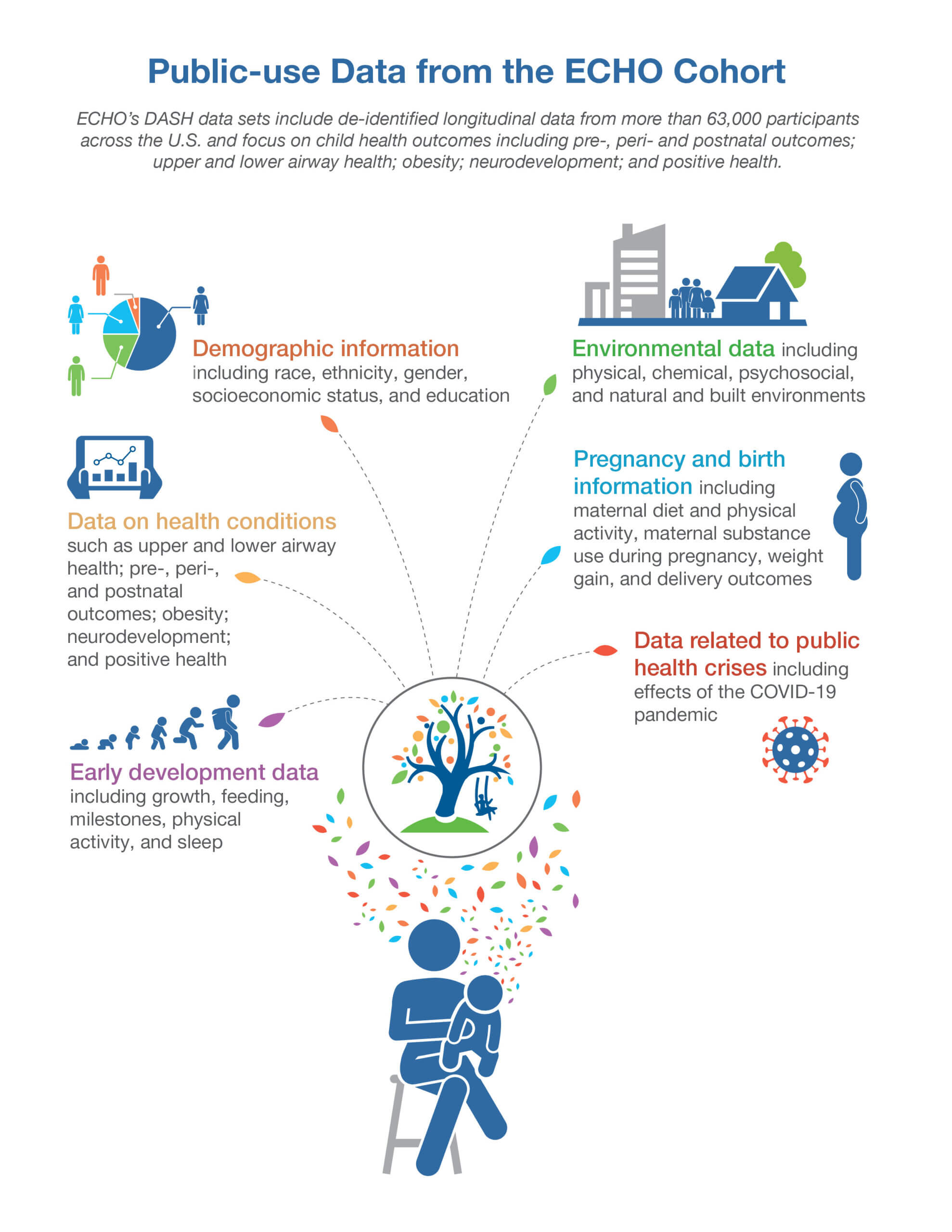
This new release includes data on 63,215 ECHO Cohort participants. This release includes extra data from participants included in the initial release and adds data on participants enrolled in the ECHO Cohort between Sept. 1, 2021, and Aug. 31, 2022. Bioassay results are newly available regarding chemicals such as flame retardants, metals and metalloids, tobacco metabolites, fungicides and herbicides, and other analytes. Additional harmonized data are also available for household chemical exposure for the child and during pregnancy, child strengths and difficulties questionnaire, perceived stress scale, caregiver general cognition, child blood pressure, early childhood education, gender identity, and public assistance.
Researchers can request access to ECHO data such as:
- Demographic information including race, ethnicity, gender, socioeconomic status, and geographic location
- Early development data including growth, milestones, physical activity, and sleep
- Environmental exposure data including physical, chemical, in utero, microbial, psychosocial, and natural and built environments
- Pregnancy and birth information including maternal diet and physical activity, maternal substance use during pregnancy, weight gain, and delivery outcomes
- Data related to public health crises including the effects of the COVID-19 pandemic
- Data on health conditions such as upper and lower airway health; pre-, peri-, and postnatal outcomes; obesity; neurodevelopment; and positive health
How does this work?
- Create a DASH account and submit a Data Request Form. If you had access to the first release of ECHO data on DASH, you will need to submit a new request for access to this release.
- The NICHD DASH Data Access Committee will review your request and respond within three weeks.
- Use the data for three years. DASH will notify you three months before your DASH Data Use Agreement expires and a renewal request can be submitted at that time.
- See the DASH Tutorial for more detailed information on the process.
Thank you for all of your efforts to support ECHO’s mission to enhance the health of children for generations to come.
Back to top
ECHO Funding Opportunities
The ECHO Program Office has released Notices of Funding Opportunities (NOFOs)
The following funding opportunities are available on the NIH ECHO website:
- RFA-OD-24-008 Clinical Sites for the Environmental influences on Child Health Outcomes (ECHO) IDeA States Pediatric Clinical Trials Network - 3 (UG1 Clinical Trial Required)
- RFA-OD-24-009 Data Coordinating and Operations Center for the ECHO IDeA States Pediatric Clinical Trials Network - 3 (U24 Clinical Trial Required—Infrastructure)
ECHO is a program funded by the NIH to enhance the health of children for generations to come. ECHO’s Institutional Developmental Award (IDeA) States Pediatric Clinical Trials Network (ISPCTN) conducts clinical trials in 18 states with historically low rates of NIH funding.
The purpose of both these NOFOs is to invite applications from entities/institutions in IDeA-eligible states* to participate in the ECHO ISPCTN, as Clinical Sites and the Data Coordinating and Operations Center (DCOC), respectively. The clinical sites and the DCOC, together, will form the ECHO ISPCTN.
The awards will support pediatric clinical trial research in the five focus areas of the ECHO Program including: pre-, peri-, and postnatal outcomes; obesity; upper and lower airways; neurodevelopment; and positive health.
The DCOC will support the Clinical Sites as they:
- Develop, conduct, and disseminate findings from multicenter clinical trials research, assuring the participation of children living in rural or underserved communities in IDeA states.
- Build pediatric clinical trial research capacity in IDeA states funded by the ECHO ISPCTN.
- Engage interested parties such as community members, nonprofit organizations, and professional societies to enhance ECHO ISPCTN clinical trial impact, transferability, rigor, and feasibility.
NIH encourages applications supporting candidates from diverse backgrounds, including those from underrepresented groups as described in the Notice of NIH's Interest in Diversity (NOT-OD-20-031).
Applications are due by April 15, 2024 by 5:00 PM local time of applicant organization. Letters of Intent are not required but are requested by April 1, 2024.
The Letter of Intent should be sent to:
Lisa Steele, PhD
Chief, Epidemiology and Population Health Branch
Division of AIDS, Behavioral and Population Sciences (DABP)
Center for Scientific Review, NIH
Phone: 301-257-2638
Email: lisa.steele@nih.gov (mailto:lisa.steele@nih.gov)
The ECHO Program Office will post Frequently Asked Questions and pre-recorded informational webinars on the NIH ECHO website as soon as they are available.
If you have questions, please reach out to ECHO ISPCTN Program Officer Tonse Raju at tonse.raju@nih.gov.
*The 23 IDeA-eligible states are Alaska, Arkansas, Delaware, Hawaii, Idaho, Kansas, Kentucky, Louisiana, Maine, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, South Carolina, South Dakota, Vermont, West Virginia, and Wyoming. The Commonwealth of Puerto Rico is also eligible.
Back to top
ECHO Research Spotlight
ECHO Research Explores Potential Value of Nutrition Data Collected from Pregnancy Through Adolescence for Understanding Child Health
Highlighting the Impact of Early-life Nutrition on Child Health
 Collaborative ECHO research led by Megan Bragg, PhD, RD; and Kristen Lyall, ScD, both of the A.J. Drexel Autism Institute highlights the opportunity for researchers to access the large amount of diet information already collected from the ECHO Cohort. This research, titled “Opportunities for Examining Child Health Impacts of Early-Life Nutrition in the ECHO Program: Maternal and Child Dietary Intake Data from Pregnancy to Adolescence,” is published in Current Developments in Nutrition.
Collaborative ECHO research led by Megan Bragg, PhD, RD; and Kristen Lyall, ScD, both of the A.J. Drexel Autism Institute highlights the opportunity for researchers to access the large amount of diet information already collected from the ECHO Cohort. This research, titled “Opportunities for Examining Child Health Impacts of Early-Life Nutrition in the ECHO Program: Maternal and Child Dietary Intake Data from Pregnancy to Adolescence,” is published in Current Developments in Nutrition.
“Early-life diet is critical for children’s growth and development. We saw this as an opportunity to highlight ECHO’s potential for researchers interested in nutrition,” said Dr. Bragg. “In order to answer questions about nutrition, researchers need information from a large, diverse group of people about what people eat during pregnancy and childhood. ECHO is unique because its research sites have collected and continue to collect this information.”
Understanding the Significance of ECHO Cohort Dietary Data in Nutrition Research
This study aimed to describe dietary intake data available in the ECHO Cohort as of August 2022, from pregnancy through adolescence, including estimated sample sizes, and to highlight the potential for future analyses of nutrition and child health. As of that date, 66 ECHO Cohort research sites across the country had collected diet information using a variety of methods, including dietary recalls, food frequency questionnaires, and questionnaires about supplement use. Due to the broad scope of research participants and because many families provided information more than once over the course of pregnancy and childhood, diet information from these research sites is especially useful.
Often, data collected on diet provide only a snapshot that can’t address how early-life diet affects later child health outcomes. The ECHO Cohort Consortium is addressing these challenges by gathering information about the dietary habits of individuals during pregnancy and childhood from ECHO Cohort participants over time.
“Prenatal and early-life nutrition has the potential to impact all five of the ECHO health outcome areas: pre-, peri- and postnatal outcomes; upper and lower airway; obesity; neurodevelopment; and positive health,” said Dr. Lyall. “Research has linked prenatal diet to everything from risk for preterm birth to children’s development of memory and attention. One domain less studied in its connection to early-life diet is positive health—this may be a promising topic for ECHO Cohort researchers.”
Leveraging ECHO Cohort Data for Future Advancements in Child Health Research
Information from over 33,000 pregnancies and more than 31,000 children in the ECHO Cohort is now accessible to researchers. These de-identified data are publicly available to researchers through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH) to encourage broad use to answer important questions about nutrition and child health.
“We hope that this study will inspire the use of dietary data from the ECHO Cohort to answer important questions in the field of early-life nutrition and child health. Researchers can use the information we’ve provided about the types of dietary data and estimated sample sizes when formulating new research questions,” said Dr. Bragg. “This study also highlights the value of the ECHO Protocol for standardizing dietary data collection going forward, enhancing comparability across ECHO Cohort Study Sites and making it easier to draw conclusions in nutrition research.”
Back to top
News You Can Use
A Qualitative Exploration of COVID-19 Vaccine Hesitancy Among Parents

ECHO ISPCTN researchers conducted a qualitative study that assessed parent/caregiver opinions on coronavirus disease 2019 (COVID-19) vaccines in children.
The goal of this study was to inform COVID-19 vaccine messaging tailored to the needs of rural, Spanish-speaking, and African American families since these groups are sometimes missed by public health messaging campaigns. Researchers recruited 36 parents and other caregivers of children who lived in rural areas, spoke Spanish, and/or identified as Black. The study population only included caregivers with at least one unvaccinated child. Researchers spoke with them about their thoughts on the COVID-19 vaccine.
Researchers gathered data in this qualitative study as preliminary work to design a mobile health application (app) intended to provide caregivers with personalized information about the COVID-19 vaccine for children. The app was subsequently tested for effect on pediatric COVID-19 vaccine uptake in the Improving Pediatric COVID-19 Vaccine Uptake Using an mHealth Tool (MoVeUP) trial. The information gathered in this preliminary qualitative study helped researchers understand what aspects of the COVID-19 vaccine were important to address in the app.
Caregivers in this study had varying opinions on trusted sources of health information, as well as questions about how the vaccine might impact their children’s health. COVID-19 vaccination decision-making was influenced by the amount and type of information available to caregivers, and caregivers’ beliefs about the possible positive and negative effects of vaccination on their children’s health, especially among caregivers whose children have medical conditions such as asthma.
“I think the findings of this article could be useful for anyone providing COVID-19 vaccine counseling for caregivers,” says study author Adrien Honcoop of University of Nebraska Medical Center. “The most fascinating thing about this study is how almost all caregivers, whether for or against [vaccinating], were willing to go to their doctor for information about the COVID-19 vaccine.”
Read about the findings in the publication “COVID-19 Vaccine Hesitancy Among Parents: A Qualitative Study,” published in the November 2023 issue of Pediatrics.
New ECHO Research Finds No Association Between Arsenic Exposure and Birth Outcomes
Collaborative ECHO research led by Jonathan Lewis, MHS; Emily Knapp, PhD, MHS; and Amii Kress, PhD, MPH of Johns Hopkins Bloomberg School of Public Health investigates the relationship between arsenic exposure and certain birth outcomes. This research, titled “Associations between area-level arsenic exposure and adverse birth outcomes: An Echo-wide cohort analysis,” is published in Environmental Research.
Researchers captured proxy exposure to arsenic using a residential history of arsenic violations from the preconception period throughout pregnancy, a time when environmental influences could disrupt fetal growth. Violations were used as a proxy for arsenic exposure. The study used data from 15,000 mother-child pairs at 51 research sites across the U.S., focusing on children born in 2006 or later. In 2006, the Environmental Protection Agency (EPA) revised the enforceable standard for arsenic concentrations in drinking water to 10 parts per billion.
Low birth weight, gestational age at birth, preterm birth, and birth size were not found to be associated with potential arsenic exposure in areas with violations. However, infants whose mothers experienced continuous exposure to arsenic from three months before conception through birth exhibited a statistically significant increase in birth weight compared to those born in areas without violations.
In future studies, researchers aim to improve the identification of the community water systems that serve ECHO Cohort participants. Future research could also consider participants’ exposure to arsenic in foods and from other sources of drinking water.
New ECHO Research Characterizes Children Born Preterm into Four Neurobehavioral Profiles Based on a Combination of Health Outcomes
 Collaborative ECHO Cohort research led by Marie Camerota, PhD of the Warren Alpert Medical School of Brown University studies the health outcomes of children born preterm and characterizes them into four neurobehavioral profiles. This research, titled “Neurodevelopmental and behavioral outcomes of very preterm infants: latent profile analysis in the Environmental influences on Child Health Outcomes (ECHO) Program,” is published in Pediatric Research.
Collaborative ECHO Cohort research led by Marie Camerota, PhD of the Warren Alpert Medical School of Brown University studies the health outcomes of children born preterm and characterizes them into four neurobehavioral profiles. This research, titled “Neurodevelopmental and behavioral outcomes of very preterm infants: latent profile analysis in the Environmental influences on Child Health Outcomes (ECHO) Program,” is published in Pediatric Research.
Birth outcomes for infants born very preterm have steadily improved over the past several decades. More children born at earlier gestational ages are surviving into childhood, however, it is unclear how being born very preterm may influence neurodevelopmental or behavioral problems.
Outcomes of children born at a gestational age of less than 33 weeks (“very pre-term”) vary significantly, with some children showing few neurodevelopmental concerns and others showing significant impairment. Most prior research has looked at single outcomes—for example, whether a child born preterm had a lower neurodevelopmental score or higher levels of behavior problems. Understanding how these different outcomes may group together can help researchers and healthcare providers provide more comprehensive treatment plans for children born very preterm.
This study included more than 2,000 babies who were born at less than 33 weeks gestational age from three ECHO Cohort Study Sites. When these children reached the age of two years, researchers conducted a neurodevelopmental assessment and a motor exam on the children while parents completed questionnaires about their children’s behavior. ECHO Cohort researchers looked for patterns in these data to understand whether there were groups of children with similar strengths and weaknesses.
Researchers found evidence for four different neurobehavioral profiles based on different combinations of cognitive, motor, and behavioral outcomes of children at the age of two years. These profiles range from few or no developmental concerns to severe impairment in one or more domains. The study placed about 85% of children into one of two groups with no/mild developmental delay and a low prevalence of behavioral problems. The remaining 15% fell into one of two profiles with more serious neurodevelopmental problems with (5%) or without (10%) co-occurring behavior problems.
“This study helps us better understand outcomes for children following a very preterm birth and shows that it is important to measure both neurodevelopmental and behavioral outcomes for children born preterm,” Dr. Camerota said. “The different groups of children we described might require different types of follow-up services or interventions. Therefore, the results of this study could potentially be used to develop personalized interventions for children following a very preterm birth.”
More research is needed to understand why some preterm children develop neurodevelopmental and/or behavioral problems and others do not. To do this, future studies may study risk factors in pregnancy, the perinatal period, and in early infancy.
Back to top
ECHO Discovery
ECHO Discovery is a monthly webinar series with educational presentations for the ECHO community and anyone else interested in environmental influences on child health.
Join Us for February ECHO Discovery!
Wednesday, February 14, 1pm ET
Untargeted Analysis of Microsamplers: The Utility of Dried Capillary Blood Spots for Exposome Research
 Dr. Lauren Petrick
Dr. Lauren Petrick
Associate Professor at Icahn School of Medicine
Director of the Center of Metabolomics and Molecular Phenotyping at Sheba Medical Center
Logistical and technical challenges in collecting venous blood specimens hinder direct biological measures during critical developmental time periods. However, dried blood microsamples, which can be collected at home or in the field and only use a finger-prick, overcome these challenges, providing new opportunities for direct biomonitoring and repeated measures in vulnerable populations, including children. Here, we will describe validation of our untargeted liquid chromatography-high resolution mass spectrometry (LC-HRMS) assay on blood microsamplers, showing the breadth of measured chemicals and metabolites compared to plasma and traditional dried blood spots on paper cards. We will also describe recently initiated pilots, highlighting microsampler use to capture exposures in time-sensitive and vulnerable populations.
January ECHO Discovery
Using Knowledge-Driven AI to Predict and Explain Environmental Health Outcomes in Mothers and Children
 Joseph D. Romano, PhD, MPhil, MA
Joseph D. Romano, PhD, MPhil, MA
Assistant Professor of Informatics, University of Pennsylvania
On January 10, Joseph Romano, PhD, MPhil, MA of the University of Pennsylvania presented for ECHO Discovery on leveraging artificial intelligence (AI) tools to predict and explain environmental health outcomes in mothers and children.
There has been a recent explosion in AI and machine learning (ML) applications that could help researchers translate large volumes of environmental health data into clinically actionable knowledge. ML is a branch of AI that seeks to create computer algorithms that can “look” at many samples of data, “learn” the data’s characteristics, and do something intelligent in response. At the University of Pennsylvania, Dr. Romano’s team is working to develop and maintain ComptoxAI—a major new data infrastructure that supports AI research in environmental toxicology. This resource combines vast amounts of data from a variety of public databases to build a graph database that can be accessed by researchers, fed into algorithms, or used to train ML models for making scientific discoveries.
AI/ML can help researchers predict how existing/novel exposure profiles may contribute to health outcomes, characterize and group similar patients, and explain the mechanisms that mediate the association between toxic exposures and disease outcomes. ECHO is uniquely positioned to provide a large volume of diverse, trans-generational, observational data on environmental exposures and social determinants of health that can be used to train robust ML models to reveal the mechanisms influencing maternal and child health outcomes.
December ECHO Discovery
Prenatal Air Pollution Exposure and Risk of Autism: New Insight from the ECHO Cohort
 Akhgar Ghassabian, MD, PhD
Akhgar Ghassabian, MD, PhD
NYU Grossman School of Medicine
NYU Langone’s Center for the Investigation of Environmental Hazards
On December 13, Akhgar Ghassabian, MD, PhD of the NYU Grossman School of Medicine and NYU Langone’s Center for the Investigation of Environmental Hazards presented for ECHO Discovery on the emerging evidence linking prenatal air pollution exposure to childhood autism spectrum disorder (ASD) risk.
Air pollution is a major environmental risk factor, and increased levels of exposure to air pollution are associated with a variety of adverse health outcomes in infancy and childhood. Emerging studies suggest prenatal and early life exposure to air pollutants may affect childhood brain development, possibly contributing to increased ASD risk.
To understand this potential link, Dr. Ghassabian and her team collected data on exposure to three different types of air pollution during pregnancy from ECHO Cohort participants across four different regions of the U.S. and evaluated the association between prenatal exposure levels and later development of childhood autism-related traits or ASD diagnosis. Overall, the research team found that prenatal exposure to certain types of pollution was associated with higher ASD risk—with some types of exposure demonstrating region-specific effects. The study also found that girls were more susceptible to the effects of prenatal air pollution, demonstrating a stronger association between exposure to certain pollutants during pregnancy and later childhood ASD risk.
Learn More about ECHO Discovery
Back to top

Subscribe to receive a copy of the ECHO Connector newsletter through email.
Questions?
Contact the ECHO Program Office.